SC-A4—A Tale of Methylchloroisothiazolinone
March 31, 2022
Hello LMS Digital News readers, and welcome back to Swiss Cheese! In case you are new to the Swiss Cheese article line, SC-A4 stands for Swiss Cheese – Article No. 4, and this month we shall discuss a student’s science-class nachtmerje (Western Frisian for “nightmare”): chemistry!
At its very core, chemistry is the study and manipulation of chemicals, but the exact definition may differ from person to person. Chemicals are not just the vile nuclear waste at power plants; they are everything, from the tiniest frog to the largest galaxy. Put simply, chemicals are matter, and since (almost) everything is composed of matter, (almost) everything is composed of chemicals. Down at an extremely microscopic level, matter is simply an arrangement of different molecules: different combinations of substances that form new materials. The pure substances that combine to form those molecules are called atoms, and the most common ones are organized in the periodic table of elements. For a long period of time, scientists believed that this was it; elements were the building blocks of the universe, until they discovered particles that were too small to be atoms…
Periodic Table of Elements (from H to Og):
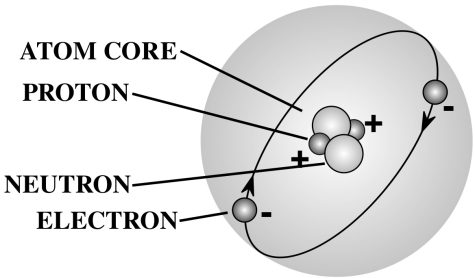
With the discovery of these “subatomic particles,” scientists were forced to rethink their ideas about the universe’s composition, and what would one day become the Standard Model of particle physics was developed as a result. This precursor to the Standard Model stated that atoms were made up of 3 subatomic particles: electrons (-1 electric charge), protons (+1 electric charge), and neutrons (no charge). Note that protons and neutrons are bound together as an atom’s nucleus (core), while the electrons orbit the nucleus from a distance away. (The diagram below only shows atomic structure conceptually; this is not a completely accurate representation of atomic structure.)
Helium-4 Atomic Structure Diagram:
Actual Atom Appearance (under powerful world-record electron microscope):
CREDIT/SOURCE: Cornell University
Very soon, scientists discovered that neutrons and protons were not fundamental particles. Further testing and experimenting revealed that neutrons and protons are composed of miniscule subsubatomic particles called quarks. These quarks functioned in peculiar ways; bonding and unbonding with other quarks to form larger subatomic particles. But the cause of their bonding
remained a mystery. Then came a monumental discovery: light was not only a wave, but also a particle. This is where quantum physics became a true science (quantum physics comes from the word quanta, a name for proposed “packets” that light traveled in). These wave-particles became known as photons, and they make up light and electromagnetism.
Formulas for energy by Albert Einstein and Max Planck:
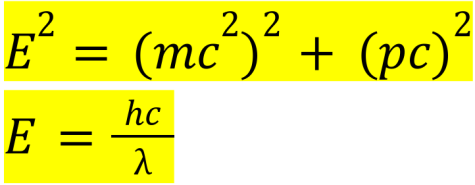
Einstein’s formula describes the relationship between E (energy in joules), m (mass in kilograms), c (speed of light in meters per second), and p (momentum in kilogram meters per second). When momentum is 0, the equation simplifies to the famous E=mc². Max Planck’s photon energy formula describes the relationship between E (energy in joules), h (Planck’s constant in joule seconds), c (speed of light in meters per second), and λ (wavelength of light in meters). This equation is slightly less known, and major applications include in the fields of photovoltaics.
With this discovery, physicists soon discovered other quantum particles pertaining to the forces of nature, collectively known as the force carriers or bosons: W & Z bosons (weak force, powers nuclear reactions), gluons (strong force, more details further on), and the legendary Higgs boson (recently discovered, somehow associated with the Higgs field). The Higgs boson/field has been determined to give mass to many other kinds of particles, but its possible links to the origin of the universe are shrouded in mystery. Remember the quark interaction mystery from earlier? The strong force can explain this through gluons. Gluons, in a sense, are like the “glue” that binds elementary particles together, in a strange quantum physical way.
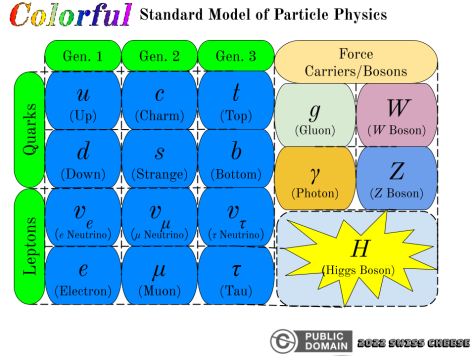
Electrons are famous for being the particle behind electricity, with a negative elementary charge, but they are not alone. Electrons are NOT quarks, and are instead part of the lepton group. This unique group contains the electron, muon, and tau particles, along with their respective neutrinos (see Standard Model diagram). Many of these particles are large (for elementary particles), and can only be found under very particular conditions.
It seems as if this article must have covered all there is to the world of particle physics, but there is so much more. From polorons to excitons, phonons to solitons, spurions to pomerons, glueballs to bradyons, there is just SO₂ (get it?) much more! Even with all this plethora of data out there, there remain some important questions that have not yet been answered. Even the Standard Model remains incomplete, with many theories out there proposing new particles (see https://home.cern/science/physics/supersymmetry for the theory of supersymmetry) to fill in the gaps. Other theories state that a graviton, a particle that also behaves as a wave (as most quantums do), is responsible for the force of gravity, one of the many forces missing from the Standard Model.
As the realm of physics expands, our knowledge of its components must do so, too. I hope that this article helped you to better understand the basics of particle sciences. If you enjoyed this article, stay tuned for the next Swiss Cheese article! (Find them all at https://lmsdigitalnews.com/?s=sc-)
Aspiring physicists or chemists? Complete the SC-A4 Quiz + Trivia to test your knowledge of the subject! (Available only to students: https://forms.gle/8MJTwQPdW5VNWTCb7) (BONUS: In the quiz, you will find out what the article’s title means!)